本文转自《The Liquid Grid》发表的题为“Ocean Storage of CO2”的文章。
作者: D. HUME · PUBLISHED
日期:2018.07.24
原文链接: http://theliquidgrid.com/2018/07/22/ocean-storage-of-co2/
Ocean Storage of CO2
Some ocean scientists are advocating for storing captured CO2 emissions in the deep ocean. The reasons why might surprise you. In this post I’ll investigate why some think ocean storage of CO2 is a good idea and the potential impacts it could have on the marine environment.
How to Stop Climate Change
Carbon dioxide (CO2) is a naturally occurring gas that plants and algae rely on for photosynthesis, and we rely on them for the oxygen we breathe. Ever since the Industrial Revolution we’ve been ejecting increasingly larger amounts of CO2 into the atmosphere, leading to a warmer planet and acidification of the ocean. No bueno for everyone on planet Earth.
If we want to mitigate climate change, there’s a very simple solution: we need to reduce the amount of carbon dioxide in the atmosphere. One strategy to rapidly decarbonize and reduce CO2 emissions is through adoption of renewable energy and improved energy efficiency, but this is not enough. Even if we somehow managed to magically stop emitting CO2 today, there is still a tremendous amount in the atmosphere that will contribute to climate change for centuries to come. In addition to decarbonization we need to remove excess CO2 from the atmosphere.
Carbon Capture and Storage
Our planet has natural carbon sinks that do this already: terrestrial plants, soil, and marine algae for example. The oceans are actually one of the largest sinks on the planet, absorbing roughly 25 percent of the atmospheric CO2 we emit each year [1]. But these natural processes take time and can’t keep up with our current level of emissions. Evidence to this fact is in the numbers: global atmospheric CO2 concentration in 1960 was 317 part per million (ppm), by 1990 was 354 ppm, by 2010 it was 390 ppm, and today it is 408 ppm [10].
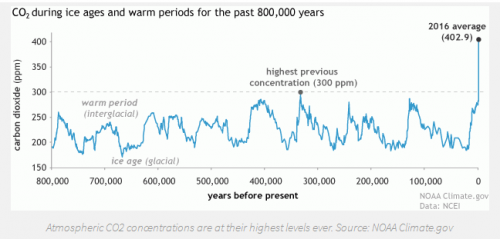
Nature needs help. We need to pull CO2 out of the atmosphere and store it away. This idea of capturing CO2 from the air and then transporting and storing it in a manmade ‘sink’ that is separated from the atmosphere is referred to as carbon capture and sequestration (or storage), known as CCS. There are many different tools and methods being researched to capture and transport carbon, but I won’t focus on those here. In this post I want to focus on CO2 sequestration, not in tanks or underground, but in the ocean.
Ocean Storage of CO2
It seems counter-intuitive: the oceans are turning more acidic due to too much CO2 and we want to put more CO2 in there? It’s a controversial idea, but there are some who think that it makes logical sense. To be clear, this is not a solution to climate change, but perhaps a way to avoid the worst of it. Let’s take a look.
Storage is required to isolate captured CO2 from the atmosphere. Traditional storage techniques trap captured CO2 as a liquid or gas deep underground in specific geological features (such as abandoned hydrocarbon wells) and require a non-porous plug to seal the injection hole and prevent release. With ocean storage of CO2, the gas is intentionally injected into the deep ocean and is either allowed to diffuse or be trapped in a specific location, depending on the depth and pressure.
There are three general methods that could be used to directly inject carbon dioxide into the ocean for storage. First, would be a pipe that runs from shore to the ocean depths. This pipe would receive a stream of carbon dioxide from a capture or intermediary storage facility on the coast and then direct it offshore for long-term storage. In the dispersal by ship method, CO2 stored on board the vessel would be ejected at great depths using a long hose or pipe towed behind the vessel, promoting rapid diffusion of the CO2 into seawater. The third method would be to use a stationary vessel or platform to inject CO2 to a fixed location at or near the ocean bottom.
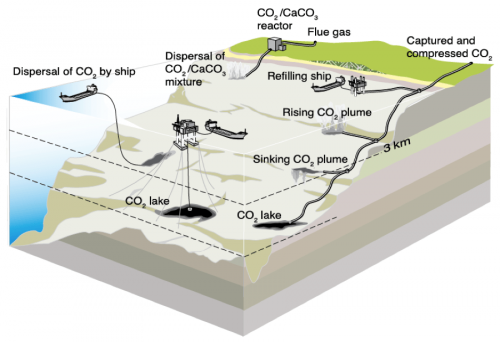
The way in which CO2 is stored in the ocean depends on its phase state and the depth at which it is injected. Remember, pressure increases with depth and we can use this fact to our advantage when it comes to ocean storage. Depending on pressure and temperature, carbon dioxide can exist as a gas, liquid, solid, or solid hydrate (a cage of water molecules encircles each CO2 molecule). From the ocean surface to depths of about 500-meters CO2 would exist as a gas and would tend to rise in the water column, since it is less dense than seawater. Between 500-meters and 2,700-meters CO2 would exist as a liquid, but one that is still lighter than seawater and would thus also rise in the water column. At depths up to 2,700-meters CO2 will create a rising plume and dissolve into the surrounding seawater relatively quickly.
At depths deeper than 3,000-meters the weight of the water column compresses the liquid CO2 and it becomes denser than seawater and sinks slowly to the seafloor. As a solid (or as a hydrate at depths below 400-meters) it will also sink, but will dissolve as it descends in the water column. Once the carbon dioxide sinks to the seafloor it forms an underwater pool at the bottom of the ocean, trapped in place by its own density.

The Benefits of Time and Space
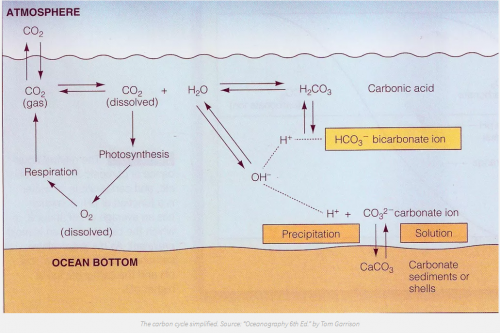
Carbon dioxide is naturally stored in the ocean through chemical processes, either as a dissolved gas or, over a longer time scale, as carbonate sediments on the seafloor. In fact, more than 70 percent of current CO2 emissions will eventually wind-up in the ocean [9]. Before the Industrial Revolution the CO2 exchange rate between the ocean and the atmosphere were pretty well balanced, but ever since we began emitting CO2the ocean has been trying to catch-up with the atmosphere. If and when our CO2 emissions ever level off it will take the atmosphere and oceans several centuries to reach equilibrium. However, this leaves tremendous amounts of CO2 in the atmosphere and upper-ocean (where it is most damaging) for hundreds of years. Storage of CO2 in the ocean accelerates this natural storage process. It transports CO2 from the atmosphere to the deep ocean, where it will eventually wind-up anyways, and do less harm.
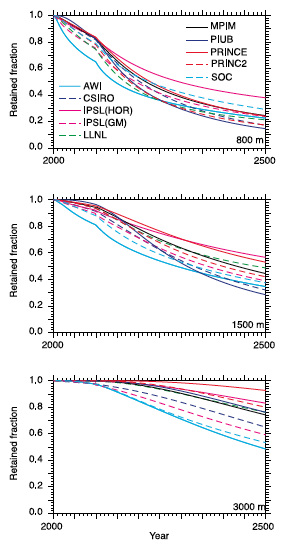
The effectiveness of ocean storage of CO2 depends on how long the stored CO2 remains isolated from the atmosphere. Ocean currents carry surface waters to the deep and vice versa. This mixing effect is more pronounced near the surface and generally decreases with depth. For seawater in the deep reaches, it can take between 300 to 1000 years for seawater to go through a complete turnover [4]. If our goal is to keep stored CO2 separated from the atmosphere as long as possible, then deeper is better. According to models, if the storage site is below 3,000-meters the fraction of CO2 that might reach the atmosphere is expected to be 20 percent over 200 years.
Another perceived advantage of ocean storage is capacity and availability. We’ve been pretty prolific with drilling holes in the ground to extract hydrocarbons. Once an oil well is emptied it might be repurposed as an underground CO2 storage reservoir, but this depends on the geology. According to the International Energy Agency’s Greenhouse Gas R&D Programme the world’s hydrocarbon reservoirs have a combined storage capacity of roughly 800 gigatonnes of CO2 (GtCO2)[5]. To put this number in perspective, the world’s annual CO2emissions are currently around 36 GtCO2 [6]. In other words, terrestrial storage could handle about 22 years worth of emissions at our current levels.
Climate change experts often refer to what is called our “carbon budget”. This is the amount of carbon that we can emit into the atmosphere without causing a two-degree Celsius temperature change. It’s perhaps one of the most important metrics to monitor since it will impact live on Earth for at least the next century. The Intergovernmental Panel on Climate Change (IPCC) helps monitor this budget and they have placed our total budget of carbon emissions at approximately one trillion tons. From 1860 to 2011 we emitted an estimated 515 billion tons of carbon, which left around 500 billion tons left in our budget eight years ago. Fast forward to today: estimates place us at values ranging from 250 to 485 billion tons remaining before we exceed our budget and catastrophically damage the earth. If our current annual emissions stay constant at 36 GtCO2 (9.8 Gt Carbon), that means we have about 25 to 50 years left before we reach the tipping point. Terrestrial storage does not have enough capacity if we continue with business as usual.
The ocean covers more than 70 percent of the planet, and roughly three-quarters of it has a depth of greater than 3,000-meters (the magic depth where storage becomes more effective). This opens up a vast amount of potential storage sites in the deep ocean. Estimates of the capacity of ocean storage range from 4,000 GtCO2 to 10,000 GtCO2 [4][9]. This is almost thirteen times the storage potential of hydrocarbon reservoirs, or about 250 years worth of storage at our current emission rate. Ocean storage of CO2 could buy us more time with our carbon budget and help us avoid peak concentrations of atmospheric CO2, perhaps avoiding the worst of climate change.
Is the Juice Worth the Squeeze?
Ocean storage of CO2 is not without environmental risks and consequences. There is the obvious issue of ocean acidification. The ocean has historically been slightly basic, averaging a pH around 8.2, though this value varies with depth. Due to the CO2 emissions over the last two centuries, the average pH has dropped 0.1 units [4][7]. While this may seem small, remember that the pH-scale is logarithmic, so this seemingly tiny change is actually a 30 percent decrease. Intentional storage may cause commensurate changes in the ocean’s pH, though it is hard to say for certain. By one calculation, adding 2,000 GtCO2 (about 50 years-worth of current emissions) via intentional storage could have a similar affect as our last two hundred years, dropping the average ocean pH another 0.1 units [9]. More CO2 in the ocean will almost certainly lead to a lower pH, the question is where in the water column and by how much.
A sharp decrease in pH creates havoc for marine creatures. Carbon dioxide in seawater leads not only to the formation of carbonic acid (which drives acidification), but also to usurping of carbonate ions from marine organisms such as lobsters, phytoplankton, and corals. These organisms rely on carbonate ions to form their exoskeletons and shells that are crucial to their survival. Some of these animals form the foundation of the marine food web; their collapse could have dire consequences.

The deep ocean is not well understood. According to NOAA, 80 percent the ocean floor remains unmapped, unobserved, and unexplored [8]. What deep-sea organisms and marine ecosystems are down there waiting to be discovered is anyone’s guess. Of course, without knowing what’s down there it’s impossible to know the environmental impacts due to high concentrations of CO2. We need a better understanding of these unique ecosystems before ocean storage of CO2 is seriously considered.
There have been a handful of experiments in the past to assess the environmental impact of ocean storage of CO2, but the few proposed in-ocean tests faced harsh public criticism forcing their abandonment [2]. This strong negative perception makes it difficult to conduct any experiments and understand the potential impacts. This means that most of the research on CO2 ocean storage is model based, but as the old adage goes: all models are wrong, but some are more useful than others. Our models make many assumptions about deep ocean circulation, mixing, and chemical properties which all need to be verified.
Ocean storage of CO2 requires choosing the lesser of two evils. The ocean is going to absorb the majority of our CO2 emissions over the coming centuries anyways, but in the process the atmosphere and ocean surface waters will be subjected to unprecedented warming and acidification. On the other hand, vast quantities of CO2 could be isolated in the deep sea, driving down atmospheric and ocean surface concentrations in the short term. This could help stabilize the climate. The cost would be untold damage to sensitive deep-sea ecosystems that we know very little about. It’s an uncomfortable choice, but it’s one that we may have to seriously consider as our carbon budget steadily dwindles.
References
[1] NOAA Pacific Marine Environmental Laboratory https://www.pmel.noaa.gov/co2/story/Ocean+Acidification
[2] Pacific Standard. “The Worlds Best Bad Idea” https://psmag.com/social-justice/ocean-carbon-sequestration-the-worlds-best-bad-idea-23521
[3] “Marine Algae as a CO2 Sink” https://link.springer.com/article/10.1007/BF00477107
[4] Chapter 6 from an IPCC Report on CCS https://www.ipcc.ch/pdf/special-reports/srccs/srccs_chapter6.pdf
[5] IEA Greenhouse Gas R&D Programme. “Storing CO2 Underground” https://ieaghg.org/docs/general_publications/storingCO.pdf
[6] Carbon Brief. https://www.carbonbrief.org/analysis-global-co2-emissions-set-to-rise-2-percent-in-2017-following-three-year-plateau
[7] Oceana. https://usa.oceana.org/what-ocean-acidification
[8] NOAA National Ocean Service. “How much of the ocean have we explored?” https://oceanservice.noaa.gov/facts/exploration.html
[9] “Ocean Storage of CO2”, E. Eric Adams and Ken Colder
https://people.ucsc.edu/~mdmccar/migrated/ocea213/readings/15_GeoEngineer/C_sequestration/adams_2008_Elements_CALDERIA_Ocean_CO2_Storeage.pdf
[10] NOAA CO2 Data ftp://aftp.cmdl.noaa.gov/products/trends/co2/co2_mm_mlo.txt
[11] Yale Environment 360. “What is the Carbon Limit? That Depends Who You Ask” November, 2014. https://e360.yale.edu/features/what_is_the_carbon_limit_that_depends_who_you_ask